Successful English Build & Language Migration
Over the past decade, the global clinical trial landscape has changed drastically. A surge in technological innovation, including wearable devices, patient portals, and apps, has modernized how clinical data is collected and managed. With pen and paper becoming obsolete in the digital era, shifting to electronic Clinical Outcome Assessments (eCOAs) is becoming more critical than ever.
However, it is imperative that the migration of COAs to an electronic format is error-free and accessible in order to collect accurate clinical data. This is particularly true for multi-regional, decentralized, and/or virtual clinical trials that require more than a reliance P&P collection of clinical data. However, migrating from paper to digital can be a complex undertaking, as linguistically validated COAs must be adapted correctly with only approved changes introduced in the migration process.
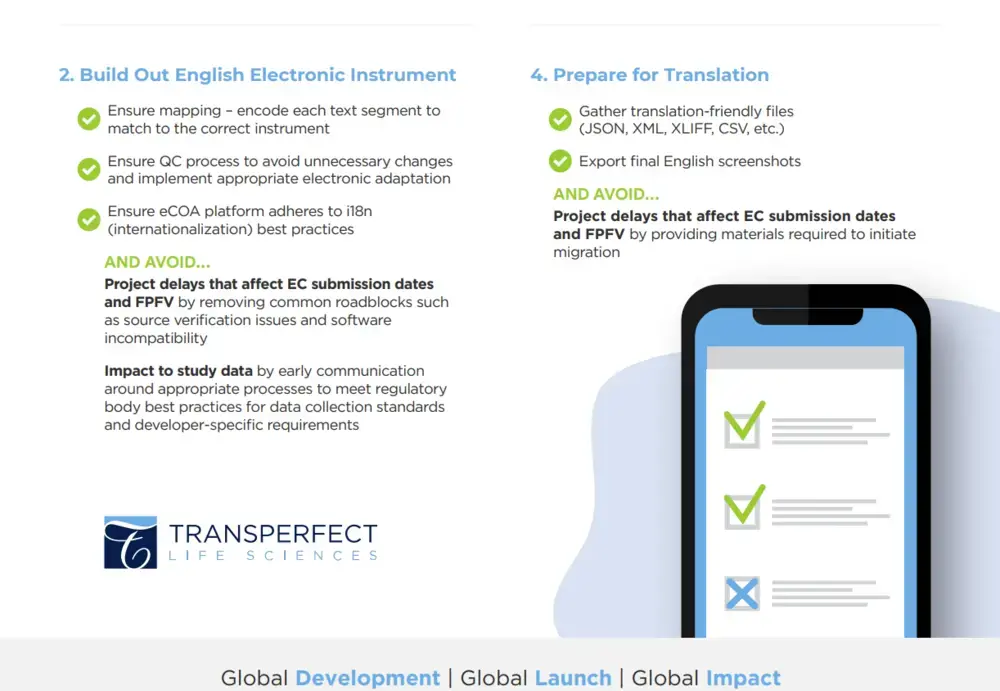
Download our checklist to guide you through the four main steps required to ensure a Successful English Build & Language Migration.

