Thought Leadership
TransPerfect Supports Takeda Supply Chain Training with Gamification
“TransPerfect supported us on building a great learning experience for our community. Elements of gaming like point scoring and competition with others are incorporated in a training to learn our KPI definitions and targets.”...
How CCMS Helps Healthcare Companies Prepare and Translate Medicare Compliance Content
The blog post discusses how healthcare companies in the United States can use a Component Content Management Solution (CCMS) to efficiently prepare and translate Medicare compliance content. ...
TransPerfect MT Earns Top Marks at International Machine Translation Competition
Read about our participation in the Sixth Conference on Machine Translation Competition. ...
Top 10 Considerations when Implementing Machine Translation (MT)
Our checklist overviews 10 essential factors to consider when implementing MT for your life sciences content, so you can leverage the benefits of increased quality, reduced costs, and shortened turnaround times this technology offers....
Language Landscape: Latin America
This post discusses the importance of localizing content for the Latin American market in the pharmaceutical industry, emphasizing the need for tailored translations due to the diverse Spanish dialects and cultural nuances across different countries, and the impact of effective...
How to Choose and Implement a CTMS
A guide for small to mid-sized biotech and medical device companies to select and implement a clinical trial management system (CTMS), discussing considerations for assessing organizational needs, technology roadmaps, vendor evaluation, and final selection to enhance clinical...
UMotif - Empowering Patients to Drive Engagement and Adherence
Watch this presentation on how empowering patients with cloud solutions increases engagement. ...
TransPerfect’s GlobalLink Helps Top-10 Pharma Meet their EC Deadlines in More than 20 Countries
A top 10 pharma company was facing delays in EC submission deadlines for their global studies....
Lessons Learned in the Rush for a Vaccine and the Effects on Language Translation: Creating and Adapting Technologies
The rush to a vaccine has accelerated an already dynamic research process. This has led to many innovations and an evolution of procedures. The language translation industry has seen enormous stressors and has had to create and modify processes and technologies similarly. The...
The Summary of Safety and Clinical Performance (SSCP) for Healthcare Professionals and Patients
An overview of the requirements and best practices for creating a Summary of Safety and Clinical Performance (SSCP) under the European Union's medical device regulation (MDR), highlighting the importance of clear, audience-specific communication for healthcare professionals...
ISPOR Europe 2020 Virtual Poster - Usability Testing for eCOAs
In order to ensure a fluid experience for a patient, usability testing is a critical data point to consider. Addressing both a patient’s needs and access to a digital platform is key to data collection. TransPerfect, along with our partners from Parexel, Clinical Ink, and...
AI Technology Speeds Safety Case Routing
TransPerfect enables a top-10 Pharma company to reduce time to route safety cases....
Medical Annotation: Using Natural Language Processing for Adverse Events
TransPerfect's DataForce Streamlines Adverse Event Detection with NLP. ...
The Paper Chase - Clinical Outcome Assessments in the Digital Age
Patients are, at last, gaining a voice in healthcare. Stakeholders have come to recognize that patients have valuable information to share about their experiences with diseases and medications that should influence policy and treatment decisions. The U.S. Food & Drug...
Artificial Intelligence in Life Sciences: Latest Developments Presented by Industry Leaders
Join TransPerfect's discussion with industry-experts from Pfizer, Bayer, CodaMetrix, Biogen, BI, Novartis, and Syneos Health about how AI is accelerating changes in the life sciences industry and ways they are leveraging data to support their teams, studies, and products. ...
Swimming in a Data Lake of eCOA Wearables
We all know there is a significant increase in data collected from wearable sensors, but what does that mean for eCOA? In our latest whitepaper , Mark Wade, Global Practice Leader for TransPerfect Life Sciences examines the considerations of adopting these sensors as an addition...
Wearables for eCOA: Practical Considerations for Improving Patient Data Capture
This electronic clinical outcome assessment (eCOA) webinar will explore the latest advancements in wearable technology and their potential to improve patient data collection in clinical studies. While there may still be ongoing discussions around deployment and standardization,...
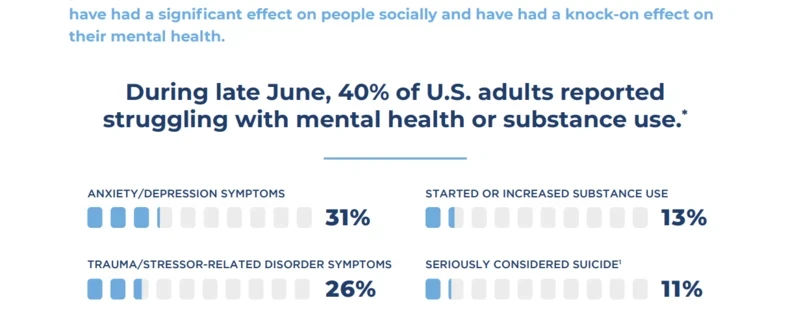
Part 3: Clinical Trials and COVID-19 Adapting to Rapid Change
The novel COVID-19 virus has forced a lot of rapid change in a very short period of time. Personally and professionally, we are all learning how to adapt. While restrictions on our day-today lives impact where, why, and how we interact, we still can come together as a community...
Part 2: Clinical Trials and COVID-19 Converting to Remote Trial Management – How to Get Started
The novel COVID-19 virus has forced a lot of rapid changes in a very short period. Personally and professionally, we are all learning how to adapt. While restrictions on our day-to-day lives impact where, why, and how we interact, we still can come together as a community to...
Part 1: Clinical Trials and COVID-19: Situation now, its challenges, and the future
The novel COVID-19 virus has forced a lot of rapid changes in a very short period. Personally and professionally, we are all learning how to adapt. While restrictions on our day-to-day lives impact where, why, and how we interact, we still can come together as a community to...
TransPerfect's OneLink Enables BI to Focus on Saving 1000 Lives
The case study highlights how TransPerfect Life Sciences helped Angelini Pharma overcome language barriers in clinical trials, enabling efficient communication and regulatory compliance....