Thought Leadership
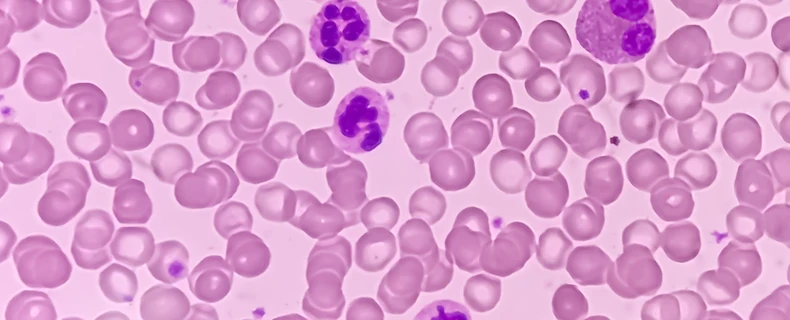
Human & Animal Blood Cell Image Annotation Spotlight
An award-winning healthcare AI company specializing in using the best in human and computational intelligence approached DataForce to build an external team with a background in biology to review and annotate thousands of microscopic images of blood smear samples. With 30,000...
How to Select the Right Regulatory Writing Company for Your Organization's Needs
Discover how clinical and regulatory teams can select the right regulatory writing and consulting company to fit their organization's needs. Learn the top five considerations when selecting the right regulatory writing and consulting company, including expertise and...
5 Essential Use Cases for Generative AI and Machine Translation in Regulatory Content Submissions
Discover how clinical and regulatory teams can mitigate risk, maintain compliance, reduce administrative timelines, and save on costs by leveraging the transformative power of generative artificial intelligence (GenAI) and machine translation workflows. Learn the five significant...
eCOA Vendor Localization Best Practices Checklist
One of the main challenges for pharma/biotech companies regarding clinical trials is prolonged eCOA study start-up. It is paramount to choose the right eCOA platform for each study. Sponsors must consider the study’s specific needs, such as user-friendliness, compliance, and...
C3 Summit Princeton 2024
Princeton Marriot Forrestal 100 College Rd E, Princeton, NJ 08540...
Selecting an eCOA Vendor to Best Fit Localization Needs
Discover how it is paramount to choose the right eCOA platform for each study. Learn the best-in-class eCOA platform localization capabilities surrounding the four core pillars of content management, internationalization, post-localization testing, and content reuse. Trust...
MedTech Summit EU 2024 | What's Past is Prologue: Evolution (and Future) of AI and Content Automation
Content automation technologies have been developing for 70 years – MDR and IVDR have accelerated this development, as well as adoption across the device industry. And, while product-related applications for artificial intelligence (AI) generate attention-grabbing headlines, the...
Pfizer x Partner4Better Spotlight
TransPerfect supports the development of the Global Learning Program: Partner4Better, by delivering relevant educational content to a global audience in diverse languages....
LifeSci Talks COA Series | Exploring the Value Add of In-Study Interviews as a Critical COA Endpoint
In this episode of TransPerfect LifeSci Talks, Mark Wade, Global Practice Leader at TransPerfect Life Sciences sits down with Tom Willgoss, Patient-Centered Outcomes Research Chapter Lead, UK Site Head for Data Sciences at Roche to discuss the value of understanding and measuring...
Artificial Intelligence in Life Sciences – Latest Developments Presented by Industry Leaders
Join TransPerfect's discussion with industry-experts from Pfizer, Bayer, CodaMetrix, Biogen, BI, Novartis, and Syneos Health about how AI is accelerating changes in the life sciences industry and ways they are leveraging data to support their teams, studies, and products. ...
AI and MT for PV and Safety Office Hours - 6/12/24
Welcome to TransPerfect Life Sciences' AI and MT Ask an Expert Office Hours. In these sessions, we provide an opportunity for our community to submit their pressing questions for our experts to answer live, in the company of your industry peers. If you are looking for some...
Strategic Artificial Intelligence (AI) Implementation for Medical Device Companies
Medical device companies are leveraging artificial intelligence and machine learning to streamline regulated medical device content work streams, including labeling, marketing, and PMS reporting. Learn about implementing a strategic AI program and discover how top medical...
Meeting the MDR & IVDR Content Challenge: New AI and Automation Solutions for New Requirements
The European Union's introduction of the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) marks a significant shift towards enhanced transparency in the medical device sector. While these regulations are designed to strengthen patient safety...
Generative AI in Life Sciences Industry: Ethics, Applications, and Transformations
Discover the role of generative artificial intelligence (GenAI) in life sciences, including what is merely a trend vs. what is truly transformative. The speakers will explore pragmatic applications, ethical considerations and the potential future role of generative AI in life...
C3 Summit 2024 - London
Conversations on Clinical Content, C3 Summit is part of a series of events hosted by TransPerfect Life Sciences featuring sessions with expert panels discussing present challenges, strategies, and innovations, as well as insights into future advancements, from R&D to...
AI and MT for Regulatory and Clinical Content Office Hours - 5/15/24
Welcome to TransPerfect Life Sciences' AI and MT Ask an Expert Office Hours. In these sessions, we provide an opportunity for our community to submit their pressing questions for our experts to answer live, in the company of your industry peers. If you are looking for some...
Trial Interactive Customer Summit, OpTImize
Sheraton Commander Hotel 16 Garden St. Cambridge, MA 02138...
AI and MT "Ask an Expert" Office Hours - 4/10/2024
TransPerfect's experts gather to answer questions from global pharma, biotech, and medical device leaders regarding AI and MT use cases, challenges, opportunities, best practices, and more....
An Overview of Generative AI and Machine Translation for Life Sciences
Global life sciences companies are realizing the potential artificial intelligence (AI) and machine learning (ML) has to drive efficiency in content and translation management. To capitalize on this, organizations need to identify the right solutions. Discover important...
6 Pathways to Take Your MT Strategy to the Next Level in 2024
With 2024 well underway, innovators in the global life sciences and pharmaceutical industries continue to question how to maximize efficiencies and reduce complexities in clinical and regulatory content development and localization. As part of the broader technological roadmap,...